Post-operative bacterial infections are a leading cause of mortality and morbidity in people who have had a liver transplant. Bacteria that cause these infections in hospitals can be resistant to a wide range of antibiotics, posing significant therapeutic challenges. Pseudomonas aeruginosa is a bacterial species that is responsible for a disproportionately high number of hospital-acquired bacterial infections, thanks in part to its ability to thrive in microbial “reservoirs” in the hospital setting. P. aeruginosa is a critical clinical pathogen due to its numerous virulence factors, ability to persist in a self-protective biofilm state, and intrinsic and acquired multi-drug resistance mechanisms. Alternative treatments for antibiotic-resistant infections are thus desperately needed.
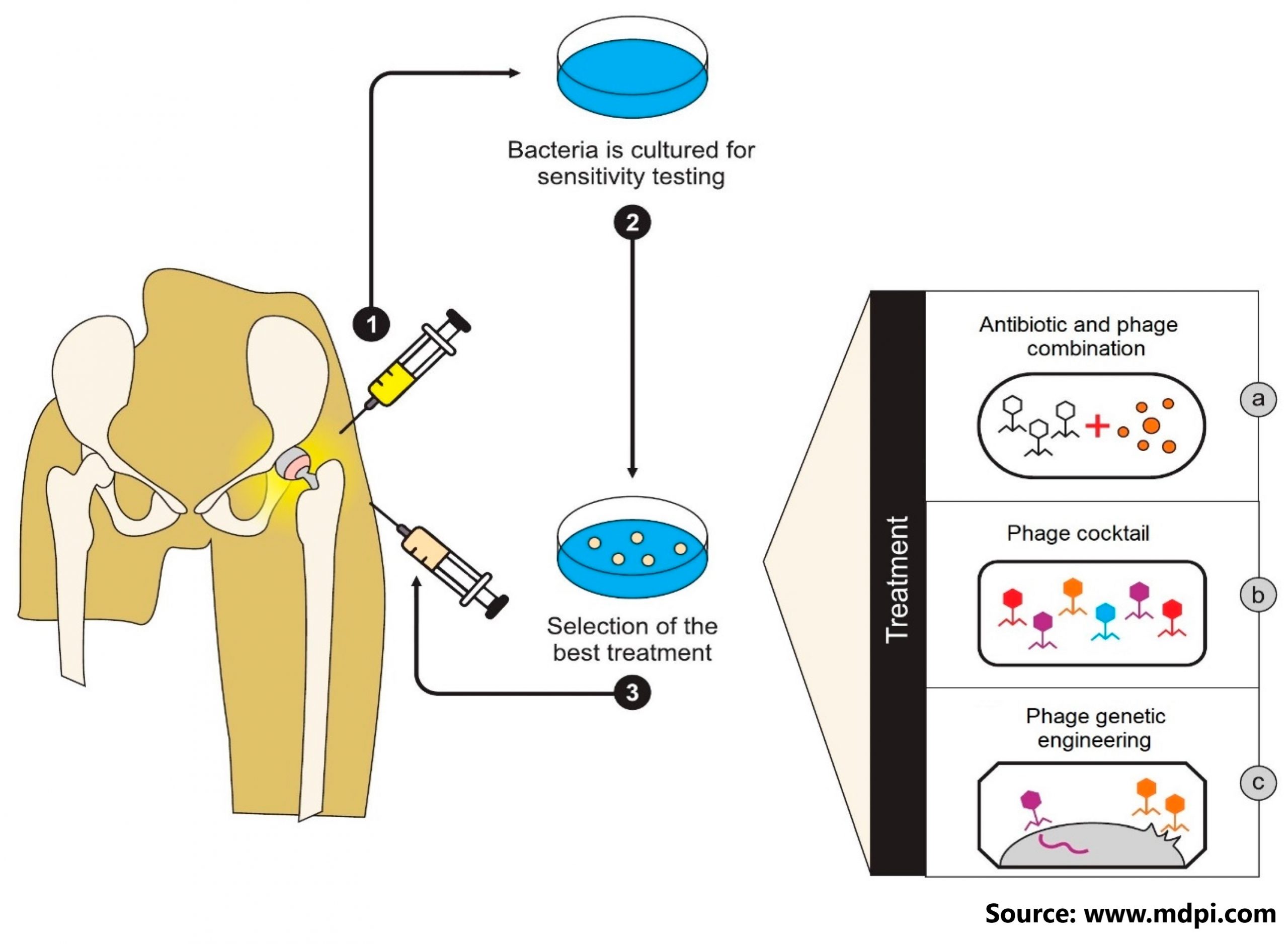
Phage therapy is one of them, and it entails using specific bacteriophage viruses – viruses that prey on bacteria and are naturally found in various environmental samples – as bactericidal agents in place of or in addition to antibiotics. The use of phage therapy raises a number of research questions in order to better understand what factors influence therapeutic success or failure. In this paper, authors tell the story of a toddler who developed drug-resistant Pseudomonas aeruginosa sepsis after a liver transplant. For 86 days, he received intravenous bacteriophage-antibiotic combination therapy. Without antibody-mediated phage neutralisation, this salvage therapy was well tolerated. It was linked to objective clinical and microbiological improvement, allowing for liver re-transplantation and the complete resolution of all infections. In vitro phage-antibiotic synergies were clearly observed. The presence of bacterial phage resistance did not result in therapeutic failure, which could be attributed to phage-induced virulence tradeoffs, which we investigated in various experimental models.
After more than two years on immunosuppressive medication, the child is doing well, with normal liver function, no transplant rejection, and no further infectious outbreaks. The colonisation of P. aeruginosa was completely cleared.
To know more, please visit https://www.nature.com/articles/s41467-022-33294-w