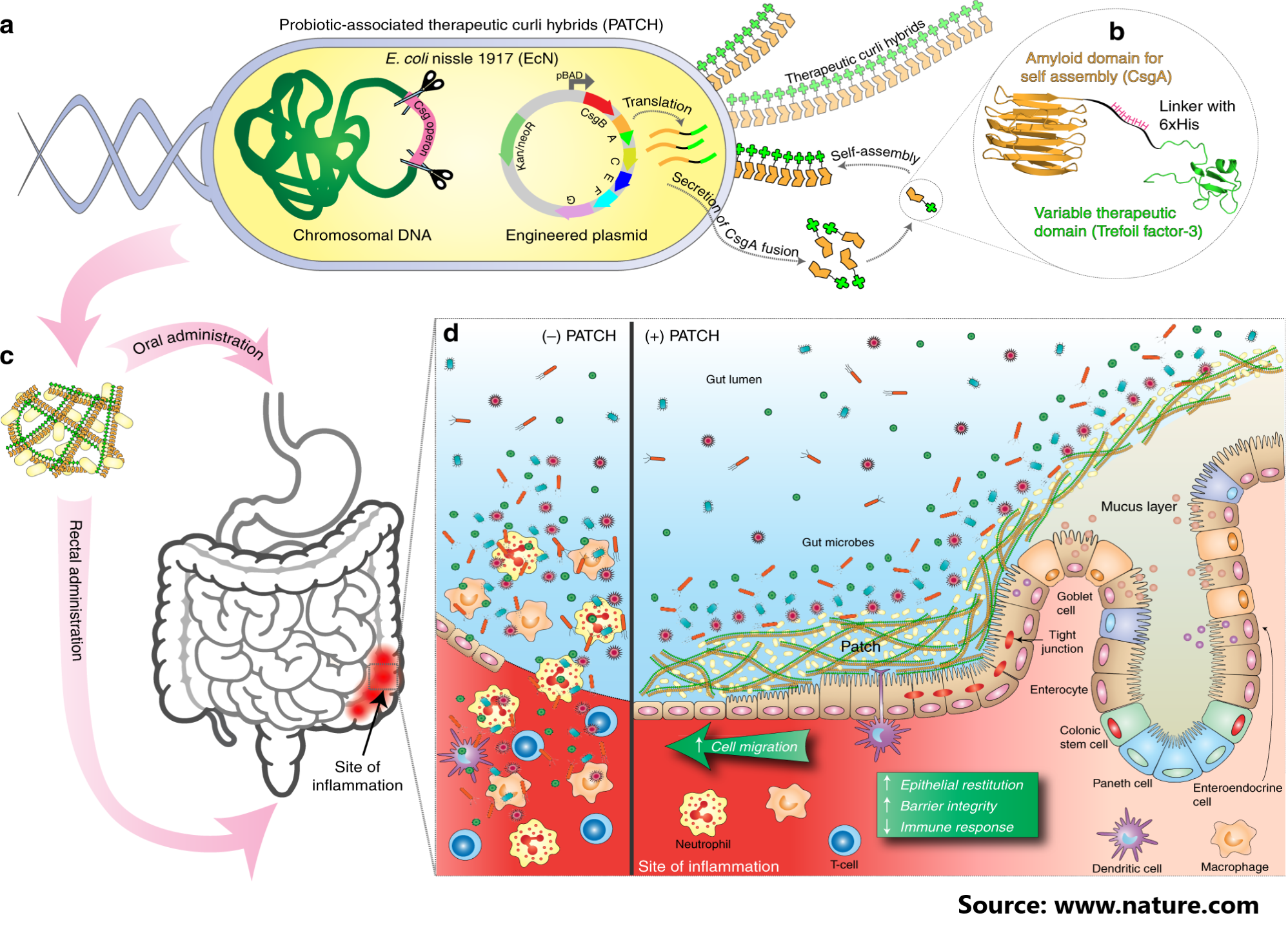
In the synthetic biology era, chemical genetics provides new tools for long-standing challenges in antimicrobial drug discovery. Microbial strains can be sensitised to drugs with specific mechanisms by making genetic changes to targeted functional pathways. High-throughput screening of these strains may then reveal new drugs that target the modified pathway specifically. Chemical genetics has recently produced drug scaffolds and assisted in the identification of drug mechanisms in a variety of microbial pathogens.
In this study, the authors conducted chemical-genetic screens in an E. coli host for targeted inhibitors of pathogen-derived enzymes. They create TESEC strains by deleting an essential E. coli enzyme and replacing it with a functionally equivalent target enzyme from a different species. The induction of the target is quantitatively controlled to determine the maximum and minimum expression levels compatible with robust growth. Target-specific inhibitors should be more effective against TESEC strains with low induction, laying the groundwork for a differential screen.
The TESEC system is designed to be as versatile and re-usable as possible with the Golden Gate assembly. Over 100 known conditionally essential E. coli metabolic genes could theoretically be supplemented with pathogen-derived analogues and TESEC-screened. Low-cost and biosafe screening technologies may enable broader participation in antibiotic discovery efforts, as well as the development of new economic models to incentivize work in this long-overlooked domain.
To know more, please visit the website of Nature (Link).