Antimicrobial resistance is a major global health issue that requires immediate attention. Notably, multidrug-resistant (MDR) Pseudomonas aeruginosa, Acinetobacter baumannii, and Enterobacterales (e.g., Klebsiella pneumoniae) are major causes of morbidity and mortality. The World Health Organization (WHO) has identified these pathogens’ carbapenem-resistant isolates as the top three priority pathogens for antibiotic drug development. Due to the current dry drug development pipeline for new antibiotics, clinicians have been forced to rely on the old polymyxin antibiotics (polymyxin B and colistin) as a last-line therapy against these difficult-to-treat Gram-negative pathogens.
Due to resistance to all other classes of antibiotics and the lean antibiotic drug development pipeline, the old polymyxin lipopeptide antibiotics (polymyxin B and colistin) are frequently the only therapeutic options. However, polymyxin B and colistin have significant safety (dose-limiting nephrotoxicity, acute toxicity), pharmacokinetics (poor pulmonary exposure), and efficacy (negligible activity against pulmonary infections) issues that have severely limited their clinical utility.
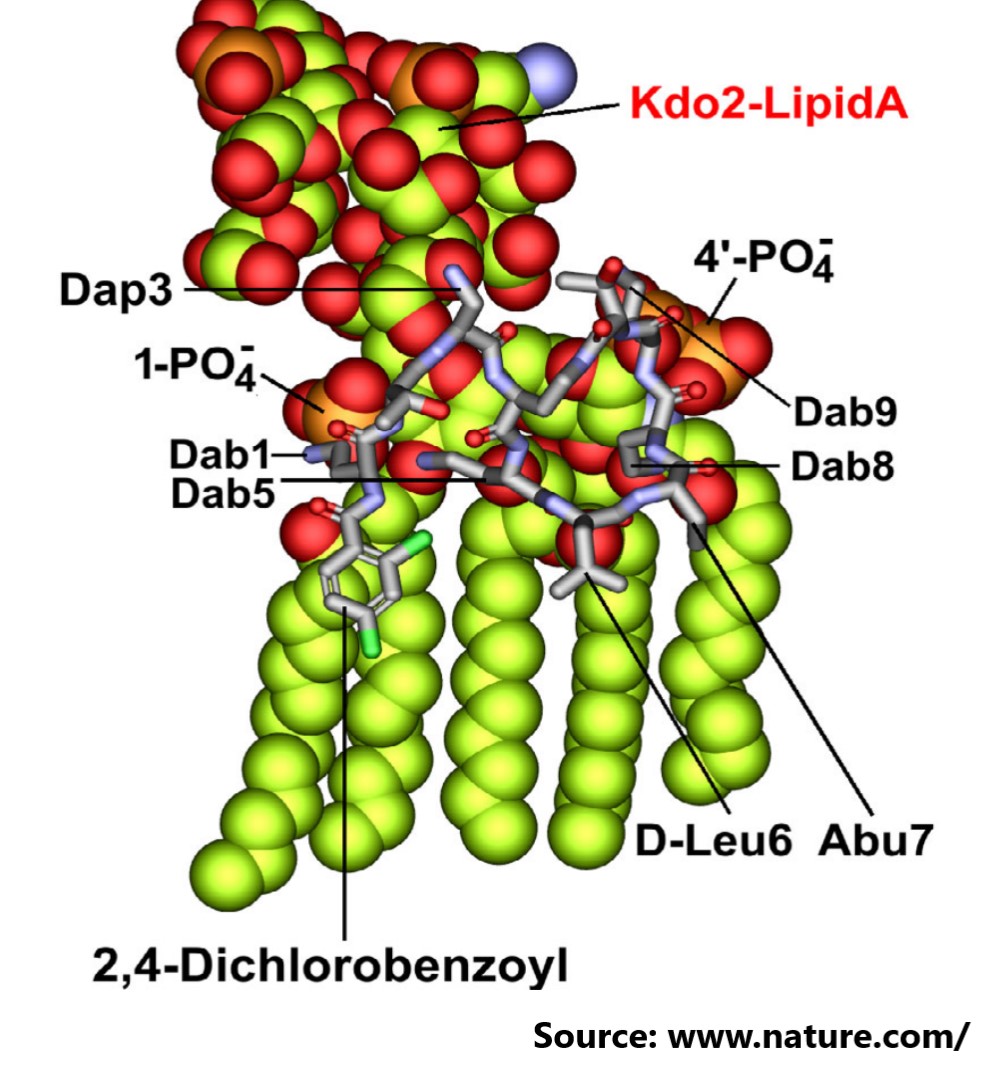
In this study, the author used chemical biology to systematically optimise multiple non-conserved positions in the polymyxin scaffold, successfully disconnecting therapeutic efficacy from toxicity, and developing a new synthetic lipopeptide that is structurally and pharmacologically distinct from polymyxin B and colistin. This resulted in the clinical candidate F365 (QPX9003), which demonstrated superior safety and efficacy against top-priority MDR pathogens Pseudomonas aeruginosa, Acinetobacter baumannii, and Klebsiella pneumoniae.
To know more, please visit the website of Nature (Link).